|
|
HOME >>
Agro
Chemicals >> Phenol Crystal

Phenol Crystal
CAS No.: 108-95-2
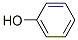
Product Identification
Synonyms: Carbolic acid; Phenic acid; Phenylic acid; Hydroxybenzene; Phenol,
fused; Monohydroxybenzene; Phenol, solid
CAS No.: 108-95-2
Molecular Weight: 94.11
Chemical Formula: C6H5OH
Product Codes:
J.T. Baker: 2858, 2862, 4056
Mallinckrodt: 0028, 0052, 0273, 0605, H602
Phenol, also known as carbolic acid, is a toxic, white crystalline solid
with a sweet tarry odor, commonly referred to as a "hospital smell". Its
chemical formula is C6H5OH and its structure is that of a hydroxyl group
(-OH) bonded to a phenyl ring; it is thus an aromatic compound.
Phenol has a limited solubility in water (8.3 g/100 ml). It is slightly
acidic: the phenol molecule has weak tendencies to lose the H+ ion from the
hydroxyl group, resulting in the highly water-soluble phenolate anion
C6H5O−, also called phenoxide anion[1][2]. Compared to aliphatic alcohols,
phenol shows much higher acidity; it even reacts with aqueous NaOH to lose
H+, whereas aliphatic alcohols do not. However, many carboxylic acids are
more acidic than phenol.
One explanation for the increased acidity over alcohols is resonance
stabilization of the phenoxide anion by the aromatic ring. In this way, the
negative charge on oxygen is shared by the ortho and para carbon atoms.[3]
In another explanation, increased acidity is the result of orbital overlap
between the oxygen's lone pairs and the aromatic system.[4] In a third, the
dominant effect is the induction from the sp2 hybridised
carbons[clarification needed]; the comparatively more powerful inductive
withdrawal of electron density that is provided by the sp2 system compared
to an sp3 system allows for great stabilization of the oxyanion. In making
this conclusion, one can examine the pKa of the enol of acetone, which is
10.9 in comparison to phenol with a pKa of 10.0.[5] However, this similarity
of acidities of phenol and acetone enol is not observed in the gas phase,
and is due to the fact that the difference of solvation energies of the
deprotonated acetone enol and phenoxide almost exactly offsets the
experimentally observed gas phase acidity difference.
It has been recently shown that only about 1/3 of the increased acidity of
phenol is due to inductive effects, with resonance accounting for the rest
Physical properties
Pure phenol is a white crystalline solid, smelling of disinfectant. It has
to be handled with great care because it causes immediate white blistering
to the skin. The crystals are often rather wet and discoloured.
Melting and boiling points
It is useful to compare phenol's melting and boiling points with those of
methylbenzene (toluene). Both molecules contain the same number of electrons
and are a very similar shape. That means that the intermolecular attractions
due to van der Waals dispersion forces are going to be very similar.

|



