|
|

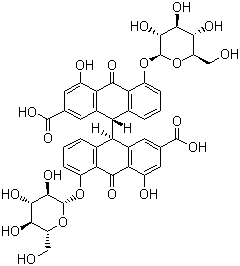
Sennoside A + B
CAS NO. 81-27-6 (Sennoside A)
128-57-4 (Sennoside B)
517-43-1, 52730-37-7 (Calcium Salt)
EINECS NO. 201-339-9 (Sennoside A)
204-895-0 (Sennoside B)
FORMULA C42H36CaO20
MOL WT. 900.81
TOXICITY
SYNONYMS Sennoside A and B Calcium salts;
5,5'-Bis(beta-D-glucopyranosyloxy)-9,9',10,10'-tetrahydro-4,4'-dihydroxy-10,10'-dioxo[9,9'-
bianthracene]-2,2'-dicarboxylic acid calcium salt
DERIVATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
PHYSICAL STATE pale brownish hygroscopic powder
MELTING POINT
BOILING POINT
SPECIFIC GRAVITY
SOLUBILITY IN WATER Soluble
pH 6.3 - 7.3
VAPOR DENSITY
AUTOIGNITION
NFPA RATINGS
Health: 2 Flammability: 0 Reactivity: 0
STABILITY
Stable under ordinary conditions. Hygroscopic.

|


