|
Chemicals
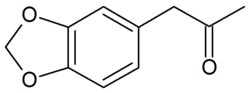
 3,4-methylenedioxyphenyl-2-propanone
3,4-methylenedioxyphenyl-2-propanone
3,4-methylenedioxyphenyl-2-propanone
CAS number [4676-39-5]
Identifiers
Molecular formula C10H10O3
CAS number [4676-39-5]
SMILES CC(=O)Cc1ccc2OCOc2c1
Properties
Molar mass 178.185 g/mol
3,4-methylenedioxy-phenyl-2-propanoneis a chemical compound
consisting of phenylacetone substituted with a methylenedioxy
functional group.
It is a chemical precursor of MDA, MDMA (more commonly known as
"Ecstasy" or "XTC"), MDEA and related chemicalsMDP2P is most
commonly synthesized by oxidizing the plant oil safrole or its
isomer isosafrole using the Wacker oxidation or peroxyacid
oxidation.
3,4-methylenedioxyphenyl)-2-propanone (MDP-2-P or PMK) was prepared
by two different routes, i.e. by oxidizing isosafrole in an acid
medium and by 1-(3,4-methylenedioxyphenyl)-2-nitropropene reduction.
The final product-MDP-2-P was subjected to GC/MS analysis.
The intermediates and reaction by-products were identified and the
‘route specific’ impurities were established.
3,4-methylenedioxyphenyl)butan-2-amine (MDP-2-MB, MBDB) is a new
homologue of N-methyl-1-(3,4-methylenedioxyphenyl)propan-2-amine (MDMA),
which is strictly controlled as a narcotic.
As part of our continuous survey on illegal designer drugs in the
Japanese market, we found that
N-methyl-4-(3,4-methylenedioxyphenyl)butan-2-amine (MDP-3-MB, HMDMA)
was being sold as MBDB.
As this is the first time that HMDMA has been revealed to be in
market distribution, and its physico-chemical data is thus far
unreported, we describe the structure elucidation of HMDMA and
comparative analysis with related compounds.
The impurity profiles were obtained by means of GC/MS, some reaction
by-products were identified by means of the EI mass spectra
including low energy EI mass spectra and ‘route specific’ impurities
were established.
4-Methyl-5-(3,4-methylenedioxyphenyl)-[1,3]dioxolan-2-one (compound
22, Table 2),
N-methyl-2-methoxy-1-methyl-2-(3,4-methylenedioxyphenyl)-ethaneamine
(compound 18, Table 2),
3-methyl-6,7-methylenedioxyisoquinoline-1,4-dione (compound 15,
Table 1) and N-cyclohexyloacetamide (compound 3, Table 1) were found
to be the synthesis markers of greatest importance.
| |
|
Note /Government
Notification: These chemicals are designated as those that are
used in the manufacture of the controlled substances and are
important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your
country government laws /control substance ACT.
Information: The information on this web page is provided to
help you to work safely, but it is intended to be an overview of
hazards, not a replacement for a full Material Safety Data Sheet
(MSDS). MSDS forms can be downloaded from the web sites of many
chemical suppliers. ,also that the information on the PTCL
Safety web site, where this page was hosted, has been copied
onto many other sites, often without permission. If you have any
doubts about the veracity of the information that you are
viewing, or have any queries, please check the URL that your web
browser displays for this page. If the URL begins "www.tajapi.com/www/Denatonium
Benzoate.htm/" the page is maintained by the Safety Officer in
Physical Chemistry at Oxford University. If not, this page is a
copy made by some other person and we have no responsibility for
it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive
Drug Abuse Prevention and Control Act of 1970.[1] The CSA is the
federal U.S. drug policy under which the manufacture,
importation, possession, use and distribution of certain
substances is regulated. The Act also served as the national
implementing legislation for the Single Convention on Narcotic
Drugs |
|
|
 |



