|
HOME >>
API >>
Dextromethorphan Hydrobromide
 .jpg)
Chemical Formulas
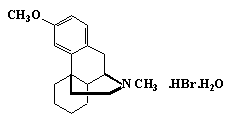
Dextromethorphan Hydrobromide

C18H25NO·HBr·H2O 370.32
Synonym:
Morphinan, 3-methoxy-17-methyl-, (9α,13α,14α)-, hydrobromide,
monohydrate.
3-Methoxy-17-methyl-9α,13α,14α- morphinan hydrobromide,
monohydrate
Identifiers
Cas No : 6700-34-1
ATC code R05DA09
PubChem CID 15978238
DrugBank APRD00655
ChemSpider 13109865 Yes
UNII 7355X3ROTS Yes
Chemical data
Formula C18H25NO
Mol. mass 271.4 g/mol
SMILES eMolecules & PubChem
InChI
Physical data
Melt. point 111 °C (232 °F)
Pharmacokinetic data
Bioavailability 11%
Molecular Weight: 352.31
Empirical Formula (Hill Notation): C18H25NO · HBr · H2O
Properties
Suitability meets USP testing specifications
Storage Temperature
Room temperature
Product
Information
This product meets USP specifications (Current through USP 23, 4th
Supplement), and is traceable to USP Reference Standard lot I
Preserve in tight containers at room temperature. Do not dry;
determine water content titrimetrically at time of use. Use
promptly. Discard unused material.
|
TEST |
USP
23 SPECIFICATIONS |
Result |
Infrared
absorption spectrum
(197K) |
compares
to standard |
conforms
to USP RS lot I |
Ultraviolet
absorption spectrum
100 μg/mL (197U) |
absorptivities at 278 nm,
calculated on the anhydrous basis, differ by not more
than 3% |
conforms to USP RS lot I |
| Identification
C |
when
dissolved 1 in 200, acidified with nitric acid, the addition of
silver nitrate causes a yellowish white precipitate to form. |
passes |
| Specific
rotation, [α]325 180 mg/10 ml solution in dioxane (781) |
specific rotation at 325 nm, calculated on the anhydrous basis,
does not differ from standard by more than 1.0% |
passes |
| pH 1
in 100 solution (791) |
between 5.2 and 6.5 |
6.5 |
| Water,
Method I (921) |
between 3.5% and 5.5% |
4.8% |
| Residue
on ignition (281) |
not
more than 0.1% |
passes |
| Limit
of N,N-dimethylaniline |
0.001% |
passes |
| Phenolic
compounds |
no
blue-green color develops |
passes |
Assay
- high pressure liquid
chromatography (621) |
not
less than 98.0% and
not more than 102.0% |
101.8% |
.jpg) *** SAMPLE AVAILABLE
*** SAMPLE AVAILABLE
Information
Associated with Product :
Dosage
Uses
Side Effects
Pharmacology


PDF DOWNLOAD
WORD DOCUMENT

 Note:
These API/ chemicals are designated as those that
are used in the manufacture of the controlled substances and are important
to the manufacture of the substances. For any (Control Substance) products
Import and Export *** subjected to your country government laws /control
substance ACT. Note:
These API/ chemicals are designated as those that
are used in the manufacture of the controlled substances and are important
to the manufacture of the substances. For any (Control Substance) products
Import and Export *** subjected to your country government laws /control
substance ACT.
Note /Government Notification:
These chemicals are designated as those that are used in the manufacture of
the controlled substances and are important to the manufacture of the
substances. For any (Control Substance) products Import and Export ***
subjected to your country government laws /control substance ACT.
Information: The information on this web page is provided to help you to
work safely, but it is intended to be an overview of hazards, not a
replacement for a full Material Safety Data Sheet (MSDS). MSDS forms can be
downloaded from the web sites of many chemical suppliers. ,also that the
information on the PTCL Safety web site, where this page was hosted, has
been copied onto many other sites, often without permission. If you have any
doubts about the veracity of the information that you are viewing, or have
any queries, please check the URL that your web browser displays for this
page. If the URL begins "www.tajapi.com" the page is maintained by the
Safety Officer in Physical Chemistry at Oxford University. If not, this page
is a copy made by some other person and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the Congress of
the United States as Title II of the Comprehensive Drug Abuse Prevention and
Control Act of 1970.[1] The CSA is the federal U.S. drug policy under which
the manufacture, importation, possession, use and distribution of certain
substances is regulated. The Act also served as the national implementing
legislation for the Single Convention on Narcotic Drugs
|


