|
|
HOME >>
API >>
API List 3 >>
Sodium Valproate
Sodium Valproate
CAS number 1069-66-5
DRUG DESCRIPTION
DOSAGE
SIDE EFFECTS
PRECAUTIONS
INTERACTION
CONSUMER INFORMATION
PDF DOWNLOAD
WORD DOCUMENT
Details

Molecular Structure
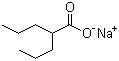
Sodium Valproate

CAS number 1069-66-5
Formula C8H15NaO2
Molecular Weight 166.19
ATC code N03AG01
PubChem 14047
ChemSpider 13428
Chemical data
Mol. mass 166.20 g/mol
Pharmacokinetic data
Bioavailability ?
Protein binding 90–95%
Metabolism 75% by CYP enzymes
Half life 9–18 hours
Excretion 20% excreted as glucuronide
- CAS number
1069-66-5
- Formula
C8H15NaO2
- Molecular
Weight 166.19
- ATC code
N03AG01
- Pub Chem
14047
- Chem Spider
13428
Microbiological Test
Total plate count MAX 1000/g
Yeast&mold MAX 100/g
E.Coli Negative
IUPAC Name: sodium 2-propylpentanoate | CAS Registry Number: 1069-66-5
Synonyms: Sodium valproate, Valproate sodium, Depakene, Epilim, Convulex,
Eurekene, Labazene, Orfiril, Valerin, Natrium valproat, Selenica, Sodium
2-propylpentanoate, Valproic acid sodium salt, Dipropylacetate sodium,
Sodium dipropylacetate, Depakene (TN), Selenica (TN), Sodium
bispropylacetate, Valproic acid sodium, Valproinsaeure, natrium
Molecular Formula: C8H15NaO2 Molecular Weight: 166.193270 [g/mol]
H-Bond Donor: 0 H-Bond Acceptor: 2
Description
Sodium valproate is indicated mainly for complex or pure absence seizure, it
can also be kinds to treat myoclonia or assist to treat it. Which is white
powder, slightly subastringent, soluble to water.
Chemistry
Sodium valproate (INN) or valproate sodium (USAN) is the sodium salt of
Valproic acid and is an anticonvulsantanticonvulsant
used in the treatment of epilepsy and bipolar disorder, as well as other
psychiatric conditions requiring the administration of a mood stabilizer.
The intravenous formulations are used when oral administration is not
possible.
- MF:
C8H15NaO2
- MW:
166.20 g/mol
-
Metabolism75% by CYP enzymes
- Half
life9-18 hours
-
Excretion: 20% excreted as glucuronide
Uses
-
Sodium
valproate is the widely used an anti-epileptic drug in the pediatric
population. Nausea, vomiting, anorexia, amenorrhoea, sedation, tremor,
weight gain, alopecia, and hepato-toxicity are the most common side
effects of this drug.The hepatotoxicity may manifest in several ways:
transient elevation of transaminase values, reversible hyperammonemia with
liver disease, or Reye syndrome - like syndrome, or progressive hepatitis.
-
Patients with
epilepsy who are on sodium valproate have been reported to have low serum
carnitine levels and this secondary carnitine deficiency has been reported
in the literature. Prolonged treatment with sodium valproate more than
other anticonvulsantsanticonvulsants,
enhances renal losses of carnitine esters, lowers serum carnitine levels,
and results in secondary carnitine deficiency.It also may cause a decrease
in serum free carnitine levels by inhibition of plasmalemmal carnitine
uptake.
Production
This
compound has been obtained by two different ways:
- The reaction
of dipropylcarbinoldipropylcarbinol
with HBr gives 4-bromoheptane, which is treated with KCN to yield
2-propylpentanenitrilepropylpentanenitrile
.Finally, this compound is hydrolyzed to the target compound with NaOH or
with H2SO4.
-
The
condensation of cyanacetic acid ethyl ester with propyl bromide by means
of sodium propoxide in propanol gives crude dipropylcyanaaceticdipropylcyanaacetic
acid ethyl ester, which, without isolation, is hydrolyzed with NaOH in hot
water yielding dipropylcyanaceticdipropylcyanacetic
acid .The decarboxylationdecarboxylation
of dipropylcyanaceticdipropylcyanacetic
acid at 140-
Safety
Profile
All
antiepilepic medications have been shown to be associated with higher risks
of fetal abnormalities (mostly for spina bifida) since at least 1983 with
the risks being related to the strength of medication used and use of more
than one drug.Valproate has also been recognised as sometimes causing a
specific facial changes ("facial phenotype") termed "fetal valproate
syndrome".Sodium valproate has been associated with the rare condition
paroxysmal tonic upgaze of childhood, also known as Ouvrier-Billson
syndrome, from childhood or fetal exposure (this condition resolved after
discontinuing valproate therapy)
Mechanism Of Toxic Effects
Valproate is a drug with anticonvulsant activity against a wide range of
seizure types and appears to be well tolerated at therapeutic doses. In
therapeutic use valproate's action may be related to altered turnover of
GABA and actions on voltage sensitive sodium channels. The mechanisms
involved in toxicity are unclear. It appears to have teratogenic effects in
therapeutic doses.

|











