
Testosterone Enanthate
Cas No 315-37-7
STORAGE
Testosterone Enanthate injection, USP should be stored at controlled
room temperature 20°C to 25°C (68°F to 77°F) [see USP].
Warming and rotating the vial between the palms of the hands will
redissolve any crystals that may have formed during storage at low
temperatures.
For Prescription Use Only
Testosterone Enanthate injection, USP provides
Testosterone Enanthate, a derivative of the primary endogenous
androgen testosterone, for intramuscular administration. In their
active form, androgens have a 17-beta-hydroxy group. Esterification
of the 17-beta-hydroxy group increases the duration of action of
testosterone; hydrolysis to free testosterone occurs in vivo. Each
mL of sterile, colorless to pale yellow solution provides 200 mg
Testosterone Enanthate in sesame oil with 5 mg chlorobutanol
(chloral derivative) as a preservative.
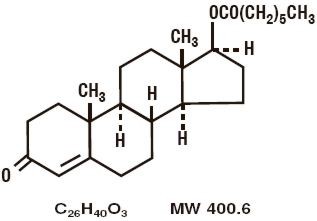
Testosterone Enanthate is designated chemically as
androst-4-en-3-one, 17-[(1-oxoheptyl)-oxy]-, (17β)-. Structural
formula:
Testosterone Enanthate - Clinical Pharmacology
Endogenous androgens are
responsible for the normal growth and development of the male sex
organs and for maintenance of secondary sex characteristics. These
effects include growth and maturation of prostate, seminal vesicles,
penis, and scrotum; development of male hair distribution, such as
beard, pubic, chest, and axillary hair; laryngeal enlargement; vocal
chord thickening; alterations in body musculature; and fat
distribution.
Androgens also cause retention of nitrogen, sodium, potassium, and
phosphorus, and decreased urinary excretion of calcium. Androgens
have been reported to increase protein anabolism and decrease
protein catabolism. Nitrogen balance is improved only when there is
sufficient intake of calories and protein.
Androgens are responsible for the growth spurt of adolescence and
for the eventual termination of linear growth which is brought about
by fusion of the epiphyseal growth centers. In children, exogenous
androgens accelerate linear growth rates but may cause a
disproportionate advancement in bone maturation. Use over long
periods may result in fusion of the epiphyseal growth centers and
termination of the growth process. Androgens have been reported to
stimulate the production of red blood cells by enhancing the
production of erythropoietic stimulating factor.
During exogenous administration of androgens, endogenous
testosterone release is inhibited through feedback inhibition of
pituitary luteinizing hormone (LH). At large doses of exogenous
androgens, spermatogenesis may also be suppressed through feedback
inhibition of pituitary follicle stimulating hormone (FSH).
There is a lack of substantial evidence that androgens are effective
in fractures, surgery, convalescence, and functional uterine
bleeding.
PHARMACOKINETICS
Testosterone esters are less polar than free testosterone.
Testosterone esters in oil injected intramuscularly are absorbed
slowly from the lipid phase; thus Testosterone Enanthate can be
given at intervals of two to four weeks.
Testosterone in plasma is 98 percent bound to a specific
testosterone-estradiol binding globulin, and about two percent is
free. Generally, the amount of this sex-hormone binding globulin (SHBG)
in the plasma will determine the distribution of testosterone
between free and bound forms, and the free testosterone
concentration will determine its half-life.
About 90 percent of a dose of testosterone is excreted in the urine
as glucuronic and sulfuric acid conjugates of testosterone and its
metabolites; about six percent of a dose is excreted in the feces,
mostly in the unconjugated form. Inactivation of testosterone occurs
primarily in the liver. Testosterone is metabolized to various
17-keto steroids through two different pathways. There are
considerable variations of the half-life of testosterone as reported
in the literature, ranging from 10 to 100 minutes.
In responsive tissues, the activity of testosterone appears to
depend on reduction to dihydrotestosterone (DHT), which binds to
cytosol receptor proteins. The steroid-receptor complex is
transported to the nucleus where it initiates transcription events
and cellular changes related to androgen action.
Indications and Usage for Testosterone
Enanthate
Males
Testosterone Enanthate injection, USP is indicated for replacement
therapy in conditions associated with a deficiency or absence of
endogenous testosterone.
Primary hypogonadism (congenital or acquired) - Testicular failure
due to cryptorchidism, bilateral torsion, orchitis, vanishing testis
syndrome, or orchidectomy.
Hypogonadotropic hypogonadism (congenital or acquired) - Idiopathic
gonadotropin or luteinizing hormone-releasing hormone (LHRH)
deficiency, or pituitary-hypothalamic injury from tumors, trauma, or
radiation. (Appropriate adrenal cortical and thyroid hormone
replacement therapy are still necessary, however, and are actually
of primary importance.)
If the above conditions occur prior to puberty, androgen replacement
therapy will be needed during the adolescent years for development
of secondary sexual characteristics. Prolonged androgen treatment
will be required to maintain sexual characteristics in these and
other males who develop testosterone deficiency after puberty.
Delayed puberty - Testosterone Enanthate injection, USP may be used
to stimulate puberty in carefully selected males with clearly
delayed puberty. These patients usually have a familial pattern of
delayed puberty that is not secondary to a pathological disorder;
puberty is expected to occur spontaneously at a relatively late
date. Brief treatment with conservative doses may occasionally be
justified in these patients if they do not respond to psychological
support. The potential adverse effect on bone maturation should be
discussed with the patient and parents prior to androgen
administration. An X-ray of the hand and wrist to determine bone age
should be obtained every six months to assess the effect of
treatment on the epiphyseal centers (see WARNINGS).
Females
Metastatic mammary cancer - Testosterone Enanthate injection, USP
may be used secondarily in women with advancing inoperable
metastatic (skeletal) mammary cancer who are one to five years
postmenopausal. Primary goals of therapy in these women include
ablation of the ovaries. Other methods of counteracting estrogen
activity are adrenalectomy, hypophysectomy, and/or antiestrogen
therapy. This treatment has also been used in premenopausal women
with breast cancer who have benefited from oophorectomy and are
considered to have a hormone-responsive tumor. Judgment concerning
androgen therapy should be made by an oncologist with expertise in
this field.
Testosterone Enanthate
Testosterone Enanthate injection, solution |
|
|
|
|
|
|



PDF DOWNLOAD
WORD DOCUMENT
Details
|

 |


