|
HOME >>
Chemicals
>>
Chemicals List 2 >> Para Anisic Acid
Para Anisic Acid
C.A.S. No. [100-09-4]
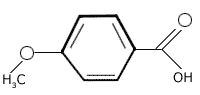
Generic Names 4-Methoxybenzoic Acid
Para Anisyl Acid
Structural Formula
Molecular Formula
Molecular Weight 152.15
C.A.S. No. [100-09-4]
Product Group Acid
Physical Characteristics
Description
Odour
Solubility - White to off white powder
- Odourless
- Alcohol, Ether and hot water
Technical Data
Melting Point
Boiling Range - 183 - 185ºC
- 195 - 205ºC
Complete Purity Profile
Purity
Moisture 98.00 % min. (HPLC)
- 0.50 % max.
Applications
• Disperse dye intermediates
• Photographic chemicals
• Pharmaceuticals Intermediates
• Repellent and Ovicide
Packing
HDPE bags with liner 25 kgs. Net.
Stability and storage
Stable for upto 6 months when stored cool and dark location
Name Fast Bordeaux GP Base
Generic Name Metanitro Pera Anicidine
4-Methoxy 2 Nitro Aniline
Molecular Weight 168
C.I. No.
37135
C.A.S. No. 96-96-8
Assay 98.5% min.
Moisture (Sample dried at 60oC) 1.0% max.
Melting Point 123° - 124°C
Appearance Very Bright Orange Red Powder
Impurities by Chromotography 2-Methoxy-5-No2 Aniline-0.2% max.
Other impurities 0.2% max.
Residue on Diazotization Does not leave more than 0.5% residue after
2 hours diazotization
Fineness (through 40 mesh screen size opening 0.42 mm) 99.8% min.
Packing 50 kgs. in fibre drums
Product Specification
|
Generic Name |
P-Anisyl
Acid
P-Methoxybenzoic Acid |
|
Melecular formula |
C8H8O8 |
|
Molecular Weight |
152.0 |
|
C.A.S. No. |
100-09-4 |
|
Purity |
99.0% Min. (GC) |
Physical Characteristics
|
Description-White to off White powder
Solubility-Soluble in Alcohol, Ether
Melting range-183.5 to 184.5ºC |
Chemical Characteristics
|
p-Methoxytoluene
- 0.06% Max.
p-Anisaldehyde - 0.06% Max.
Moisture - 0.5% Max. |
|
Packing |
Fibre / Mild Steel Drums 25/50 kgs Net. |
Application
Disperse Dye Intermediates
Repellent And Ovicide
Photographic Chemicals
Stability
and Storage
Stable for up to 6 months when stored in
full, tightly sealed containers in a cool location.
Precaution
Exposure to skin and eye may cause
irritation. Inhalation of vapours may irritate nose, throat and
lungs. Wash skin exposure with water and decontaminate the areas
by rubbing not less than 5 minutes. Flood eyes with water for not
less than 10 minutes. If the patient stops breathing apply
artificial respiration and administer oxygen. In case of
accidental ingestion induce vomiting and contact physician.
| |
|
Note /Government
Notification: These chemicals are designated as those that are
used in the manufacture of the controlled substances and are
important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your
country government laws /control substance ACT.
Information: The information on this web page is provided to
help you to work safely, but it is intended to be an overview of
hazards, not a replacement for a full Material Safety Data Sheet
(MSDS). MSDS forms can be downloaded from the web sites of many
chemical suppliers. ,also that the information on the PTCL
Safety web site, where this page was hosted, has been copied
onto many other sites, often without permission. If you have any
doubts about the veracity of the information that you are
viewing, or have any queries, please check the URL that your web
browser displays for this page. If the URL begins "www.tajapi.com/www/Denatonium
Benzoate.htm/" the page is maintained by the Safety Officer in
Physical Chemistry at Oxford University. If not, this page is a
copy made by some other person and we have no responsibility for
it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive
Drug Abuse Prevention and Control Act of 1970.[1] The CSA is the
federal U.S. drug policy under which the manufacture,
importation, possession, use and distribution of certain
substances is regulated. The Act also served as the national
implementing legislation for the Single Convention on Narcotic
Drugs |
|
|
 |



