|
|

Pantoprazole Sodium
CAS number 138786-67-1
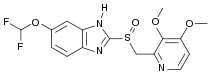
Systematic (IUPAC) name
6-(difluoromethoxy)-2-[(3,4-dimethoxypyridin-2-yl)methylsulfinyl]-1H-benzo[d]imidazole
Identifiers
CAS number 138786-67-1
ATC code A02BC02
PubChem 4679
DrugBank APRD00073
ChemSpider 4517
Chemical data
Formula C16H15F2N3O4S
Mol. mass 383.371 g/mol
SMILES eMolecules & PubChem
Pharmacokinetic data
Bioavailability 77%
Metabolism Hepatic (CYP2C19 and 3A4)
Half life 1 hour
Excretion Renal
Therapeutic considerations
Pregnancy cat.
B3(AU) B(US)
Legal status
Prescription only
Routes Oral and intravenous
Pantoprazole is a proton pump inhibitor drug used for short-term treatment
of erosion and ulceration of the esophagus caused by gastroesophageal reflux
disease. Initial treatment is generally of eight weeks' duration, after
which another eight week course of treatment may be considered if necessary.
It can be used as a maintenance therapy for long term use after initial
response is obtained. This medication may affect the results of certain lab
tests, such as drug screenings (pantoprazole can cause a false positive for
THC). It is recommended you make sure laboratory personnel and your doctor
know you are using this drug. The active ingredient in (pantoprazole
sodium) Delayed-Release Tablets is a substituted benzimidazole , sodium
5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl] sulfinyl]-1 H -benzimidazole
sesquihydrate, a compound that inhibits gastric acid secretion.

|


