|
HOME >>
API >>
Tramadol

Tramadol cas No. 27203-92-5

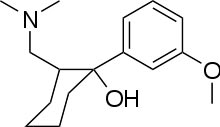 Systematic
(IUPAC) name Systematic
(IUPAC) name
(1R,2R)-rel-2-[(dimethylamino)methyl]-
1-(3-methoxyphenyl)cyclohexanol
Identifiers
CAS number : 27203-92-5
ATC code : N02AX02
PubChem : 33741
DrugBank : APRD00028
ChemSpider : 31105
Chemical data
Formula : C16H25NO2
Mol. mass : 263.4 g/mol
SMILES : eMolecules & PubChem
Pharmacokinetic data
Bioavailability 68–72% Increases with repeated dosing.
Protein binding 20%
Metabolism Hepatic demethylation and glucuronidation
Half life 5–7 hours
Excretion Renal
Tramadol is a centrally acting analgesic, used for treating
moderate to severe pain. It is often categorized as an opioid,
although it is chemically not related to the opioid class of drugs.
It does, however, appear to have agonist actions at the μ-opioid
receptor as well as the noradrenergic and serotonergic systems.
Tramadol is used to treat moderate to moderately severe pain and
most types of neuralgia, including trigeminal neuralgiaIt has been
suggested that tramadol could be effective for alleviating symptoms
of depression, anxiety, and phobias because of its action on the
noradrenergic and serotonergic systems.However, health professionals
have not yet fully endorsed of its use on a large scale for these
disorders, although it may be used when other treatments have
failed.
 Tramadol is a centrally acting analgesic. The chemical name for
tramadol hydrochloride is
(±)cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyr) cyclohexanol
hydrochloride.Tramadol hydrochloride is a white, bitter, crystalline
and odorless powder. It is readily soluble in water and ethanol and
has a pKa of 9.41. The n-octanol/water log partition coefficient (logP)
is 1.35 at pH 7. Tramadol is a centrally acting analgesic. The chemical name for
tramadol hydrochloride is
(±)cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyr) cyclohexanol
hydrochloride.Tramadol hydrochloride is a white, bitter, crystalline
and odorless powder. It is readily soluble in water and ethanol and
has a pKa of 9.41. The n-octanol/water log partition coefficient (logP)
is 1.35 at pH 7.
How should this medicine be used?
Tramadol comes as a tablet and an extended-release (long-acting)
tablet to take by mouth. The regular tablet is usually taken with or
without food every 4-6 hours as needed. The extended-release tablet
should be taken once a day. Take the extended-release tablet at
about the same time of day every day, and either always take it with
food or always take it without food. Take tramadol exactly as
directed. Do not take more medication as a single dose or take more
doses per day than prescribed by your doctor. Taking more tramadol
than prescribed by your doctor may cause serious side effects or
death.
Your doctor may start you on a low dose of tramadol and gradually
increase the amount of medication you take, not more often than
every 3 days if you are taking the regular tablets or every 5 days
if you are taking the extended-release tablets.
Swallow the extended-release tablets whole; do not split, chew, or
crush them. Do not snort (inhale powder from crushed tablet) or
inject the dissolved extended-release tablets. Taking this
medication in a way that is not recommended may cause serious side
effects or death.
Tramadol can be habit-forming. Do not take a larger dose, take it
more often, or take it for a longer period of time than prescribed
by your doctor. Call your doctor if you find that you want to take
extra medication or if you notice any other unusual changes in your
behavior or mood.
Tramadol side effects
Get emergency medical help if you have any of these signs of an
allergic reaction: hives; difficulty breathing; swelling of your
face, lips, tongue, or throat. Stop using tramadol and call your
doctor at once if you have any of these serious side effects:
* seizure (convulsions);
* a red, blistering, peeling skin rash; or
* shallow breathing, weak pulse.
Less serious side effects may include:
* dizziness, drowsiness, weakness;
* nausea, vomiting, constipation, loss of appetite;
* blurred vision;
* flushing (redness, warmth, or tingly feeling); or
* sleep problems (insomnia).
Availability
Tramadol is usually marketed as the hydrochloride salt (tramadol
hydrochloride); the tartrate is seen on rare occasions, and tramadol
is available in both injectable (intravenous and/or intramuscular)
and oral preparations. It is also available in conjunction with
acetaminophen. The solutions suitable for injection are used in
Patient-Controlled Analgesia pumps under some circumstances, either
as the sole agent or along with another agent such as morphine.
Tramadol comes in many forms, including:
* capsules
* tablets
* extended-release tablets
* extended-release capsules
* chewable tablets
* low-residue and/or uncoated tablets that can be taken by the
sublingual and buccal routes
* suppositories
* effervescent tablets and powders
* ampoules of sterile solution for SC, IM, and IV injection
* preservative-free solutions for injection by the various spinal
routes (epidural, intrathecal, caudal, and others)
* powders for compounding
* liquids both with and without alcohol for oral and sublingual
administration, available in regular phials and bottles, dropper
bottles, bottles with a pump similar to those used with liquid soap
and phials with droppers built into the cap
* tablets and capsules containing paracetamol (acetaminophen) and
aspirin and other agents
Tramadol has been experimentally used in the form of an ingredient
in multi-agent topical gels, creams, and solutions for nerve pain,
rectal foam, concentrated retention enaema, and a skin plaster (transdermal
patch) quite similar to those used with lidocaine.
Veterinary Use
Tramadol is used to treat post-operative, injury-related, and
chronic (e.g., cancer-related) pain in dogs and cats [8] as well as
rabbits, coatis, many small mammals including rats and flying
squirrels, guinea pigs, ferrets, and raccoons. Tramadol comes in
ampoules in addition to the tablets, capsules, powder for
reconstitution, and oral syrups and liquids; the fact that its
characteristic taste is not very bitter and can be masked in food
and diluted in water makes for a number of means of administration.
No data that would lead to a definitive determination of the
efficacy and safety of tramadol in reptiles or amphibians is
available at this time, and, following the pattern of all other
drugs, it appears that tramadol can be used to relieve pain in
marsupials such as North American opossums, Short-Tailed Opossums,
sugar gliders, wallabies, and kangaroos among others.
Metabolism
Tramadol undergoes hepatic metabolism via the cytochrome P450
isozyme CYP2D6, being O- and N-demethylated to five different
metabolites. Of these, M1 (O-Desmethyltramadol) is the most
significant since it has 200 times the μ-affinity of (+)-tramadol,
and furthermore has an elimination half-life of nine hours, compared
with six hours for tramadol itself. In the 6% of the population that
have slow CYP2D6 activity, there is therefore a slightly reduced
analgesic effect. Phase II hepatic metabolism renders the
metabolites water-soluble, which are excreted by the kidneys. Thus,
reduced doses may be used in renal and hepatic impairment.

 Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT. Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Note /Government Notification:
These chemicals are designated as those that are used in the
manufacture of the controlled substances and are important to the
manufacture of the substances. For any (Control Substance) products
Import and Export *** subjected to your country government laws
/control substance ACT.
Information: The information on this web page is provided to
help you to work safely, but it is intended to be an overview of
hazards, not a replacement for a full Material Safety Data Sheet (MSDS).
MSDS forms can be downloaded from the web sites of many chemical
suppliers. ,also that the information on the PTCL Safety web site,
where this page was hosted, has been copied onto many other sites,
often without permission. If you have any doubts about the veracity
of the information that you are viewing, or have any queries, please
check the URL that your web browser displays for this page. If the
URL begins "www.tajapi.com/www/Denatonium Benzoate.htm/" the page is
maintained by the Safety Officer in Physical Chemistry at Oxford
University. If not, this page is a copy made by some other person
and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive Drug
Abuse Prevention and Control Act of 1970.[1] The CSA is the federal
U.S. drug policy under which the manufacture, importation,
possession, use and distribution of certain substances is regulated.
The Act also served as the national implementing legislation for the
Single Convention on Narcotic Drugs.
|
|



 Systematic
(IUPAC) name
Systematic
(IUPAC) name  Tramadol is a centrally acting analgesic. The chemical name for
tramadol hydrochloride is
(±)cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyr) cyclohexanol
hydrochloride.Tramadol hydrochloride is a white, bitter, crystalline
and odorless powder. It is readily soluble in water and ethanol and
has a pKa of 9.41. The n-octanol/water log partition coefficient (logP)
is 1.35 at pH 7.
Tramadol is a centrally acting analgesic. The chemical name for
tramadol hydrochloride is
(±)cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyr) cyclohexanol
hydrochloride.Tramadol hydrochloride is a white, bitter, crystalline
and odorless powder. It is readily soluble in water and ethanol and
has a pKa of 9.41. The n-octanol/water log partition coefficient (logP)
is 1.35 at pH 7.








