|
HOME >>
API >>
Codeine Base

|
Codeine base

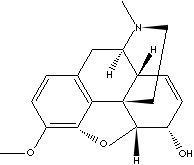
|
|
PRODUCT IDENTIFICATION
CAS NO. :
76-57-3 (Base) 1422-07-7 (Hydrochloride)
FORMULA :
C18H21NO3
MOL WT. :
317.38
DERIVATION TOXICITY : Oral rat LD50: 427 mg/kg
SYNONYMS
Methyl morphine monohydrate;
7,8-Didehydro-4,5-alpha-epoxy-3-methoxy-17-methylmorphinan-
6-alpha-ol; 3-o-methylmorphine Monohydrate;
7,8-Didehydro-4,5-epoxy-3-methoxy- 17-methyl-(5alpha,
6alpha)-morphinan 6-ol; Actacode; Calcidrine; Codicept; Codalgin
Forte Codate; (-)-Codeine; Codeinum; Coducept; Metilmorfina;
Morphine-3-methyl ether; Morphine monomethyl ether; N-Methyl
Norcodine;
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
PHYSICAL STATE :
white crystalline powder
MELTING POINT :
154 - 156 C
BOILING POINT :
250 C
SPECIFIC GRAVITY :
1.32
SOLUBILITY IN WATER : pH
VAPOR DENSITY :
AUTOIGNITION
NFPA RATINGS
Health: 3 Flammability: 1 Reactivity: 1
FLASH POINT :
75 C
STABILITY :
Stable under ordinary conditions
Odor :
None found
Taste :
None found
DESCRIPTION
Codeine is an alkaloid found naturally in opium in
concentrations ranging from 0.7 to 2.5 percent. It is
commercially synthesized from morphine. It is the most widely
used drug among the morphine class of compounds used in a
variety of pharmaceuticals including analgesics,
antiperistaltics, antitussive agents, hypnotics, and sedatives.
Codeine is an opioid analgesic related to morphine but with less
potential analgesic property and mild sedative effect.
Dihydrocodeine, called also drocode, is a similar opioid
analgesic with pharmaceutical action with codeine. It is known
it has approximately twice potentiality.
SPECIFICATION :
APPEARANCE
white crystalline needles
PURITY
99.0% min (Dry Basis)
OPTICAL ROTATION
-106° ~ -110° (C=1 in water)
WATER
10.0% max
TRANSPORTATION PACKING
HAZARD CLASS UN NO.
OTHER INFORMATION
Hazard Symbols: , Risk Phrases: 20/21/22, Safety Phrases: 26-36 |
Codeine is an alkaloid found in opium and other poppy
saps like Papaver bracteatum, the Iranian poppy, in concentrations
ranging from 0.3 to 3.0 percent. While codeine can be extracted from
opium, most codeine is synthesized from morphine through the process
of O-methylation. It was first isolated in 1832 in France by
Jean-Pierre Robiquet.
The effects of the Nixon War On Drugs by 1972 or so had caused
across-the-board shortages of illicit and licit opiates because of a
scarcity of natural opium, poppy straw and other sources of opium
alkaloids, and the geopolitical situation was getting less helpful
for the United States. After a large percentage of the opium and
morphine in the US National Stockpile of Strategic & Critical
Materials had to be tapped in order to ease severe shortages of
medicinal opiates—the codeine-based antitussives in particular—in
late 1973, researchers were tasked with and quickly succeeded in
finding a way to synthesize codeine and its derivatives and
precursors from scratch from petroleum or coal tar using a process
developed at the United States' National Institutes of Health.
Numerous codeine salts have been prepared since the drug was
discovered. The most commonly used are the hydrochloride (freebase
conversion ratio 0.805), phosphate (0.736), sulphate (0.859) and
citrate (0.842). Others include a salicylate NSAID, codeine
salicylate (0.686), and at least four codeine-based barbiturates,
the cyclohexenylethylbarbiturate (0.559),
cyclopentenylallylbarbiturate (0.561), diallylbarbiturate (0.561),
and diethylbarbiturate (0.619).
Uses
Codeine is an opiate used for its analgesic, antitussive, and
antidiarrheal properties.
Codeine is useful for numbing back pain, and is frequently
prescribed for this purpose.
Codeine is by far the most widely used opiate in the world and
probably the most commonly used drug overall according to numerous
reports over the years by organizations such as the World Health
Organization and its League of Nations predecessor agency and
others. It is one of the most effective orally-administered opioid
analgesics and has a wide safety margin.
Safety Profile
A human poison by an unspecified route. An experimental poison by
ingestion, intraperitoneal, intravenous, intramuscular, and
subcutaneous routes. Human reproductive effects. An experimental
teratogen. Other experimental reproductive effects. An addictive
drug. Flammable when exposed to heat or flame. To fight fire, use
alcohol foam. When heated to decomposition it emits toxic fumes of
NOx.
Hazards Identification
DANGER! MAY BE FATAL IF SWALLOWED. HARMFUL IF INHALED OR ABSORBED
THROUGH SKIN. ALLERGEN. EXPOSURE MAY PRODUCE ALLERGIC RESPONSE.
NARCOTIC.
Inhalation:
Narcotic. Can irritate the respiratory passages and cause sneezing
or coughing but will also have an anesthetic effect. Inhalation of
appreciable quantities may produce lung edema, dizziness, and
respiratory difficulties, see also Ingestion, below.
Ingestion:
Toxic. Narcotic. Human lethal dose about 800 mg. In addition to its
analgesic action, codeine can cause slow respiration, cyanosis, weak
pulse, gastrointestinal spasm, pinpoint pupils and twitching or
convulsions. Death from respiratory failure can occur.
Skin Contact:
Not expected to cause health effects, although the possibility of
absorption exists under conditions of skin breakage or inflammation.
Eye Contact:
Mild irritant but will also have a strong narcotic effect (pupil
constriction) and the eye may serve as an absorption route to the
body in general.
Chronic Exposure:
Can lead to habituation and addiction. Pinpoint pupils and rapid
changes of mood may be observed.
Aggravation of Pre-existing Conditions:
Some individuals may become sensitized from exposure and develop
skin rashes, coughs, stuffy nose, asthma, and other allergic
complaints. Sensitivity may develop soon after immediate contact or
after years of exposure.
First Aid Measures
Inhalation:
Remove to fresh air. If not breathing, give artificial respiration.
If breathing is difficult, give oxygen. Call a physician.
Ingestion:
Induce vomiting immediately as directed by medical personnel. Never
give anything by mouth to an unconscious person.
Skin Contact:
Immediately flush skin with plenty of water for at least 15 minutes
while removing contaminated clothing and shoes. Call a physician,
immediately. Wash clothing before reuse.
Eye Contact:
Immediately flush eyes with plenty of water for at least 15 minutes,
lifting lower and upper eyelids occasionally. Get medical attention
immediately.
Fire Fighting Measures
Fire:
As with most organic solids, fire is possible at elevated
temperatures or by contact with an ignition source.
Explosion:
Not considered to be an explosion hazard.
Fire Extinguishing Media:
Water spray, dry chemical, alcohol foam, or carbon dioxide.
Special Information:
In the event of a fire, wear full protective clothing and NIOSH-approved
self-contained breathing apparatus with full facepiece operated in
the pressure demand or other positive pressure mode.
 Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT. Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Note /Government Notification:
These chemicals are designated as those that are used in the
manufacture of the controlled substances and are important to the
manufacture of the substances. For any (Control Substance) products
Import and Export *** subjected to your country government laws
/control substance ACT.
Information: The information on this web page is provided to
help you to work safely, but it is intended to be an overview of
hazards, not a replacement for a full Material Safety Data Sheet (MSDS).
MSDS forms can be downloaded from the web sites of many chemical
suppliers. ,also that the information on the PTCL Safety web site,
where this page was hosted, has been copied onto many other sites,
often without permission. If you have any doubts about the veracity
of the information that you are viewing, or have any queries, please
check the URL that your web browser displays for this page. If the
URL begins "www.tajapi.com/www/Denatonium Benzoate.htm/" the page is
maintained by the Safety Officer in Physical Chemistry at Oxford
University. If not, this page is a copy made by some other person
and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive Drug
Abuse Prevention and Control Act of 1970.[1] The CSA is the federal
U.S. drug policy under which the manufacture, importation,
possession, use and distribution of certain substances is regulated.
The Act also served as the national implementing legislation for the
Single Convention on Narcotic Drugs.
|


|














