|
HOME >>
API >>
Methylphenidate

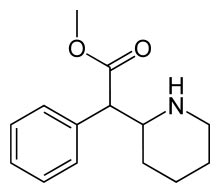 Methylphenidate Methylphenidate

Systematic (IUPAC) name
methyl phenyl(piperidin-2-yl)acetate
Identifiers
CAS number : 113-45-1
ATC code : N06BA04
PubChem : 4158
DrugBank : APRD00657
ChemSpider : 4015
Chemical data
Formula : C14H19NO2
Mol. mass : 233.31 g/mol
SMILES : eMolecules & PubChem
Pharmacokinetic data
Bioavailability 11–52%
Protein binding 30%
Metabolism Liver
Half life 2–4 hours
Excretion Urine
Therapeutic considerations
Pregnancy cat. C
Routes Oral, Transdermal, IV, Nasal
Methylphenidateis the most commonly prescribed psychostimulant and
is indicated in the treatment of attention-deficit hyperactivity
disorder, Postural Orthostatic Tachycardia Syndrome and narcolepsy,
although off-label uses include treating lethargy, depression,
neural insult, and obesity. In North America it is most commonly
known as the brand name Ritalin, which is an instant-release racemic
mixture, although a variety of brand names and formulations
exist.Methylphenidate is a potent central nervous system stimulant
derived from amphetamine, and is thought to exert its effect by
increasing dopaminergic stimulation in the brain.
History
Methylphenidate was patented in 1954 by the CIBA pharmaceutical
company (now Novartis) as a potential cure for Mohr's disease
Beginning in the 1960s, it was used to treat children with ADHD or
ADD, known at the time as hyperactivity or minimal brain dysfunction
(MBD). Today methylphenidate is the most commonly prescribed
medication to treat ADHD around the world.Production and
prescription of methylphenidate rose significantly in the 1990s,
especially in the United States, as the ADHD diagnosis came to be
better understood and more generally accepted within the medical and
mental health communities.
Attention deficit hyperactivity disorder
Methylphenidate is approved by the FDA for the treatment of
attention-deficit hyperactivity disorderThe addition of behavioural
modification therapy (e.g. CBT) has additional benefits on treatment
outcomeThere is a lack of evidence of the effectiveness in the long
term of beneficial effects of methylphenidate with regard to
learning and academic performance.A meta analysis of the literature
concluded that methylphenidate quickly and effectively reduces the
signs and symptoms of ADHD in children under the age of 18 in the
short term but found that this conclusion may be biased due to the
high number of low quality clinical trials in the literature. There
have been no placebo controlled trials investigating the long term
effectiveness of methylphenidate beyond 4 weeks thus the long term
effectiveness of methylphenidate has not been scientifically
demonstrated. Serious concerns of publication bias regarding the use
of methylphenidate for ADHD has also been noted.A diagnosis of ADHD
must be confirmed and the benefits and risks and proper use of
stimulants as well as alternative treatments should be discussed
with the parent before stimulants are prescribed.The dosage used can
vary quite significantly from individual child to individual child
with some children responding to quite low doses whereas other
children require the higher dose range. The dose therefore should be
titrated to an optimal level which achieves therapeutic benefit and
minimal side effectsTherapy with methylphenidate should not be
indefinite. Weaning off periods to assess symptoms are recommended.
Pregnancy Implications
There are no well-controlled studies establishing safety in pregnant
women. Animal studies have shown teratogenic effects to the fetus.
Do not use in women of childbearing age unless the potential benefit
outweighs the possible risk.
Lactation
Excretion in breast milk unknown/use caution
 Contraindications Contraindications
Hypersensitivity to methylphenidate, any component of the
formulation, or idiosyncrasy to sympathomimetic amines; marked
anxiety, tension, and agitation; glaucoma; use during or within 14
days following MAO inhibitor therapy; Tourette's syndrome or tics
Warnings/Precautions:
Has demonstrated value as part of a comprehensive treatment program
for ADHD. Safety and efficacy in children <6 years of age not
established. Use with caution in patients with bipolar disorder,
diabetes mellitus, cardiovascular disease, hyperthyroidism, seizure
disorders, insomnia, porphyria, or hypertension. Use caution in
patients with history of ethanol or drug abuse. May exacerbate
symptoms of behavior and thought disorder in psychotic patients. Do
not use to treat severe depression or fatigue states. Potential for
drug dependency exists - avoid abrupt discontinuation in patients
who have received for prolonged periods. Visual disturbances have
been reported (rare). Stimulant use has been associated with growth
suppression. Growth should be monitored during treatment. Stimulants
may unmask tics in individuals with coexisting Tourette's syndrome.
should not be used in patients with esophageal motility disorders or
pre-existing severe gastrointestinal narrowing (small bowel disease,
short gut syndrome, history of peritonitis, cystic fibrosis, chronic
intestinal pseudo-obstruction, Meckel's diverticulum).
Stability
Immediate release tablet: Do not store above 30°C (86°F); protect
from light Extended release capsule: Store in dose pack provided at
25°C (77°F) Sustained release tablet: Do not store above 30°C
(86°F); protect from moisture Osmotic controlled release tablet
Store at 25°C (77°F); protect from humidity
Mechanism of Action
Mild CNS stimulant; blocks the reuptake mechanism of dopaminergic
neurons; appears to stimulate the cerebral cortex and subcortical
structures similar to amphetamines
Methylphenidate is a medication prescribed for individuals (usually
children)
who have attention-deficit hyperactivity disorder (ADHD), which
consists of a
persistent pattern of abnormally high levels of activity,
impulsivity, and/or
inattention that is more frequently displayed and more severe than
is typically
observed in individuals with comparable levels of development. The
pattern of
behavior usually arises between the ages of 3 and 5, and is
diagnosed during
the elementary school years due to the child’s excessive locomotor
activity, poor attention, and/or impulsive behavior.
Most symptoms improve during adolescence or adulthood, but the
disorder can
persist or present in adults. It has been estimated that 3–7 percent
of school-age children have ADHD. Methylphenidate also is
occasionally prescribed for
treating narcolepsy.
Health Effects
Methylphenidate is a central nervous system (CNS) stimulant. It has
effects
similar to, but more potent than, caffeine and less potent than
amphetamines. It has a notably calming and “focusing” effect on
those with ADHD, particularly
children.
 Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT. Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Note /Government Notification:
These chemicals are designated as those that are used in the
manufacture of the controlled substances and are important to the
manufacture of the substances. For any (Control Substance) products
Import and Export *** subjected to your country government laws
/control substance ACT.
Information: The information on this web page is provided to
help you to work safely, but it is intended to be an overview of
hazards, not a replacement for a full Material Safety Data Sheet (MSDS).
MSDS forms can be downloaded from the web sites of many chemical
suppliers. ,also that the information on the PTCL Safety web site,
where this page was hosted, has been copied onto many other sites,
often without permission. If you have any doubts about the veracity
of the information that you are viewing, or have any queries, please
check the URL that your web browser displays for this page. If the
URL begins "www.tajapi.com/www/Denatonium Benzoate.htm/" the page is
maintained by the Safety Officer in Physical Chemistry at Oxford
University. If not, this page is a copy made by some other person
and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive Drug
Abuse Prevention and Control Act of 1970.[1] The CSA is the federal
U.S. drug policy under which the manufacture, importation,
possession, use and distribution of certain substances is regulated.
The Act also served as the national implementing legislation for the
Single Convention on Narcotic Drugs.
|


|




 Methylphenidate
Methylphenidate
 Contraindications
Contraindications








