|
HOME >>
Chemicals
>>
Chemicals List 2 >> 2-butanone (or methyl ethyl ketone)
2-butanone (or methyl ethyl ketone)
CAS number 78-93-3
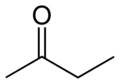

 Identifiers Identifiers
CAS number [78-93-3]
RTECS number EL6475000
SMILES CCC(=O)C
Properties
Molecular weight: 72.1057
Molecular formula C4H8O
Molar mass 72.11 g/mol
Appearance Colorless liquid
Density 0.8050 g/cm³
Melting point -86 °C, 187 K, -123 °F
Boiling point 79.6 °C, 353 K, 175 °F
Solubility in water 27.5%
Viscosity 0.43 cP at 20 °C
Structure
Dipole moment 2.76 D
Hazards
MSDS External
MSDS EU classification Flammable (F) Irritant (Xi)
R-phrases R11, R36, R66, R67
S-phrases (S2), S9, S16
Flash point −9 °C
Autoignition temperature 505 °C
LD50 6.86 ml/kg (oral, rat)
2-Butanone, also known as methyl ethyl ketone (MEK), is a colorless
liquid with a sweet, but sharp odor. 2-Butanone is manufactured in
large amounts for use in paints, glues, and other finishes because
it rapidly evaporates and will dissolve many substances. It will
quickly evaporate into the air. 2-Butanone is often found dissolved
in water or as a gas in the air. 2-Butanone is also a natural
product made by some trees and is found in some fruits and
vegetables.
The exhausts of cars and trucks release 2-butanone into the air.
2-Butanone is usually found in the air, water, and soil of landfills
and hazardous waste sites.
In water, 2-butanone can be changed to a more simple chemical form
by natural biological processes and will be broken down in about 2
weeks. It will not be deposited in the sediment of rivers or lakes,
and it is not expected to concentrate in fish. In air, 2-butanone
will break down under the influene of sunlight, although it does not
react with sunlight directly.
One-half of any given amount of 2-butanone in the air will break
down in 1 day or less. It is not known if 2-butanone changes to a
more simple form by natural biological processes in the soil, but it
is expected to do so because similar substances are broken down by
these processes.
2-Butanone will not stick to soil, and if it is spilled onto soil,
it will travel through the soil into underground water sources. Some
of the 2-butanone found in soil or water will also evaporate to the
air.
Exposure
2-Butanone can enter the environment in a number of different ways.
It can enter the air or water from the waste of manufacturing
plants. 2-Butanone is present in many different types of paints and
glues used both in the home and in industry.
As these products dry, 2-butanone will enter the air. 2-Butanone is
also in air because it is released in the exhaust of cars and
trucks. Some trees in the forest release 2-butanone to the air.
We do not know the background levels of 2-butanone in air, water, or
soil. We know that 2-butanone is found naturally in some foods. We
know it is found at hazardous waste sites, and it is also found
occasionally in drinking water and often in the air of cities. You
may also be exposed to 2-butanone by smoking cigarettes.
You may be exposed to higher levels of 2-butanone if you use glues
or coatings containing it in a small enclosed area that does not
have good air flow.
People who use it at work have a good chance of being exposed to
2-butanone. 2-Butanone is used in such industries as shoe factories,
printing plants, plastics factories, and sporting goods
manufacturers.
People who live near a toxic waste site where 2-butanone is kept may
breathe it if it evaporates into the air, or drink it if it gets
into the water supply, especially when the water supply come from
wells.
Metabolism
2-Butanone can enter your body if you breathe air that contains it,
through your skin if it touches you, or through your mouth if you
eat food or drink water that has 2-butanone in it. Studies have
shown that, if there is 2-butanone in the air you breathe, at least
half of what you breathe in will enter your body.
The other half will leave in the air you breathe out. We do not know
how much 2-butanone will stay in your body if you drink it or if it
touches your skin. The amount of 2-butanone that actually enters
your body depends on how much is in the air you breathe, how much is
in your food or water, or how much gets on your skin.
The amount of 2-butanone that enters your body also depends on how
long you breathe it or how long it is on your skin before you wash
it off. Your body gets rid of 2-butanone in urine and in the air you
breathe out. 2-butanone is not a chemical that stays in your body
for very long; it will be gone by the next day.
Health Effects
Some people who breathed air that contained 2-butanone first noticed
its sweet, sharp odor at a concentration of 5-8 parts of 2-butanone
per million parts of air (5-8 ppm). The main health effects that
have been seen in humans who breathed higher concentrations of
2-butanone are mild irritation of the nose, throat, eyes, and skin.
Serious health effects in animals have been seen only at very high
concentrations of 2-butanone. These high concentrations are not
expected in the usual use of 2-butanone or in the vicinity of
hazardous waste sites. Studies in animals have shown that 2-butanone
does not cause serious damage to the nervous system or the liver,
but mice that breathed low levels for a short time had temporary
behavioral effects. 2-Butanone alone does not have serious effects
on the liver or nervous system, but it can cause other chemicals to
become more harmful to these systems.
Guinea pigs, rats, and mice that breathed high levels of 2-butanone
for a short time became unconscious and died. Pregnant rats and mice
that breathed air containing high levels of 2-butanone had
underdeveloped fetuses.
The rats that swallowed very high concentrations of 2-butanone in
water also developed signs of nervous system effects such as
inactivity, drooping eye lids, and uncoordinated muscle movement.
Some rats and mice that swallowed water containing high
concentrations of 2-butanone died. Rats that received water
containing a lower concentration of 2-butanone had mild kidney
damage. Skin irritation developed in rabbits and guinea pigs that
had small amounts of 2-butanone dropped on their skin. Rabbits that
had small amounts of 2-butanone dropped in their eyes had serious
eye irritation.
We do not know whether 2-butanone causes birth defects or affects
reproduction in humans. Reproductive effects were not seen in
animals exposed to 2-butanone. We have no information about whether
2-butanone causes cancer in humans or animals.
| |
|
Note /Government
Notification: These chemicals are designated as those that are
used in the manufacture of the controlled substances and are
important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your
country government laws /control substance ACT.
Information: The information on this web page is provided to
help you to work safely, but it is intended to be an overview of
hazards, not a replacement for a full Material Safety Data Sheet
(MSDS). MSDS forms can be downloaded from the web sites of many
chemical suppliers. ,also that the information on the PTCL
Safety web site, where this page was hosted, has been copied
onto many other sites, often without permission. If you have any
doubts about the veracity of the information that you are
viewing, or have any queries, please check the URL that your web
browser displays for this page. If the URL begins "www.tajapi.com/www/Denatonium
Benzoate.htm/" the page is maintained by the Safety Officer in
Physical Chemistry at Oxford University. If not, this page is a
copy made by some other person and we have no responsibility for
it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive
Drug Abuse Prevention and Control Act of 1970.[1] The CSA is the
federal U.S. drug policy under which the manufacture,
importation, possession, use and distribution of certain
substances is regulated. The Act also served as the national
implementing legislation for the Single Convention on Narcotic
Drugs |
|
|
 |



