|
HOME >>
Chemicals
>>
Chemicals List 2 >> Methyl Tyramine
Methyl Tyramine
CAS NO. 55-81-2
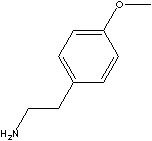
PRODUCT IDENTIFICATION
CAS NO. 55-81-2
O-METHYL TYRAMINE
EINECS NO. 200-245-5
FORMULA C9H13NO
MOL WT. 151.21
H.S. CODE
TOXICITY
SYNONYMS 4-Methoxyphenethylamine;
p-Methoxyphenethylamine; p-Methoxyphenylethylamine; Homoanisylamine;
2-(p-Methoxyphenyl)ethylamine;
2-(4-Methoxyphenyl)ethylamine; 4-Methoxy-beta- phenylethylamine;
4-Methoxy-2-phenethylamine; 4-Methoxybenzeneethanamine;
4-Methoxyphenethylamine; 4-Methoxyphenylethylamine;
2-(4-Methoxyphenyl)ethanamine; p-metoxifenetilamina (Spanish);
p-méthoxyphénéthylamine
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
PHYSICAL STATE pale yellow clear liquid
MELTING POINT
BOILING POINT 138 - 140 C at 20 mm Hg
SPECIFIC GRAVITY 1.03 - 1.035
SOLUBILITY IN WATER
pH
VAPOR DENSITY
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
1.538
FLASH POINT
STABILITY
Stable under ordinary conditions
APPLICATIONS
Tyramine [4-(2-Aminoethyl)phenol] is a monoamine compound of
4-hydroxy phenethylamine. It is derived from tyrosine, an amino acid
(protein building block) that is the precursor of norepinephrine.
It, through its effect on neurotransmitters, may affect several
health conditions, including Parkinson's disease, depression,
alcohol withdrawal support, and Phenylketonuria. (PKU). Tyramine is
naturally found in plant and animal tissues, certain cheeses and
ergot or produced synthetically. It is metabolized by monoamine
oxidase. Tyramine or its derivatives can be used in pharmaceutical
industry such as:
* Sympathomimetic or adrenergic drugs themselves or intermediate of
them.
* Intermediate for bezafibrate used in the treatment of high
cholesterol levels.
* Determination of peroxide activity in the fluorescence enzyme
immunoassay for insulin.
* False transmitter.
Tryptamine [3-(2-Aminoethyl)indole] is a biologically important
monoamine compound derived by the decarboxylation of tryptophan,
indole side chain amino acid. In addition to its function to build
the structure of protein, tryptophan is a precursor for
neurotransmitter and neurohormone. Tryptamine (biological monoamine)
effects vasoconstriction by causing the release of norepinephrine at
postganglionic nerve endings. Several mental disorders and health
conditions are explained as due to either an excess or deficiency of
monoamines. There are many natural tryptamines in both animal and
plant as well as synthetics modified by chemical constituents and
substituted at appropriate positions in the motif. Common tryptamine
class compounds include;
* Bufotenine (CAS #: 487-93-4): (5-Hydroxy-N,N-dimethyltryptamine,
pressor agent, epinephrine-like.
* DMT (CAS #: 61-50-7): N,N-Dimethyltryptamine, a psychedelic and
hallucinogenic.
* Luzindole (CAS #: 117946-91-5): N-Acetyl-2-benzyltryptamine
* Melatonin (CAS #: 73-31-4, 8041-44-9):
N-Acetyl-5-methoxytryptamine, biological clock hormone.
* Psilocin: 4-Hydroxy-N,N-dimethyltryptamine.
* Psilocybin (CAS #: 520-52-5):
4-Phosphoryloxy-N,N-dimethyltryptamine, a hallucinogenic.
* Serotonin (CAS #: 50-67-9): 5-Hydroxytryptamine, a
vasoconstrictor.
* Sumatriptan( CAS #: 103628-46-2)
4-Hethylaminosulfonyl-N,N-dimethyltryptamine, selective serotonin
antagonist used in the treatment of migraine headache.
SPECIFICATION
APPEARANCE
pale yellow clear liquid
ASSAY
99.0% min
TRANSPORTATION
PACKING 200kgs in fiber drum
HAZARD CLASS 8 (Packing Group: III)
UN NO. 2735
OTHER INFORMATION
Hazard Symbols: C, Risk Phrases: 34, Safety Phrases: 26-36/37/39-45
GENERAL DESCRIPTION OF CATECHOLAMINE
Catecholamine: a group of naturally occurring sympathomimetic amines
that have important physiological functions within the body as
neurotransmitters in the central nervous system and hormones in the
blood circulation. Catecholamines are biogenic amines considered as
sympathomimetic drugs; They are characterized by a catechol group
[The ortho (1,2) isomer of dihydroxybenzene] to which is attached an
amine group (the aromatic portion of whose molecule is catechol, and
the aliphatic portion an amine). The most abundant catecholamines in
the body are epinephrine (adrenaline), norepinephrine (noradrenaline)
and dopamine. They are derived from the tyrosine, an amino acid
(protein building block) that is the precursor of norepinephrine.
(The prefix nor- describes normal structure which has no branched
chain of carbon atoms. In case of norepinephrine, it has one less
methylene group than its homologue, epinephrine.) Catecholamines
belong to a broader class of compounds called phenethylamines which
contain structurally amino acid, phenylalanine and tyrosine.
Phenethylamine is a backbone for the compounds which take roles of
alkaloids as well as hormones and neurotransmitters in nature.
Amphetamine is the substituted phenethylamine by methyl group at
alpha position. It is a synthetic drug used as a diet suppressant
and to treat narcolepsy and ADHD (attention deficit hyperactivity
disorder). But amphetamines can produce severe psychological
dependence, including cardiac irregularities and gastric
disturbances. Chronic use often results in extreme exhaustion and
malnutrition.
| |
|
Note /Government
Notification: These chemicals are designated as those that are
used in the manufacture of the controlled substances and are
important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your
country government laws /control substance ACT.
Information: The information on this web page is provided to
help you to work safely, but it is intended to be an overview of
hazards, not a replacement for a full Material Safety Data Sheet
(MSDS). MSDS forms can be downloaded from the web sites of many
chemical suppliers. ,also that the information on the PTCL
Safety web site, where this page was hosted, has been copied
onto many other sites, often without permission. If you have any
doubts about the veracity of the information that you are
viewing, or have any queries, please check the URL that your web
browser displays for this page. If the URL begins "www.tajapi.com/www/Denatonium
Benzoate.htm/" the page is maintained by the Safety Officer in
Physical Chemistry at Oxford University. If not, this page is a
copy made by some other person and we have no responsibility for
it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive
Drug Abuse Prevention and Control Act of 1970.[1] The CSA is the
federal U.S. drug policy under which the manufacture,
importation, possession, use and distribution of certain
substances is regulated. The Act also served as the national
implementing legislation for the Single Convention on Narcotic
Drugs |
|
|
 |



