|
HOME >>
Chemicals
>>
Chemicals List 2 >>
Acetic anhydride
Acetic anhydride
CAS number 108-24-7
Product Identification
Synonyms: Acetyl oxide; Acetic acid anhydride; Acetic oxide;
Ethanoic anhydride
CAS No.: 108-24-7
Molecular Weight: 102.09
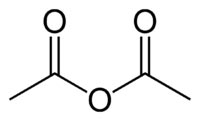
Chemical Formula: (CH3CO)2O
Product Codes:
J.T. Baker: 0018
Mallinckrodt: 2420
Hazards Identification
DANGER! CORROSIVE. CAUSES BURNS TO ANY AREA OF CONTACT. FLAMMABLE
LIQUID AND VAPOR. WATER REACTIVE. HARMFUL IF SWALLOWED OR INHALED.
VAPOR CAUSES RESPIRATORY TRACT IRRITATION AND SEVERE EYE IRRITATION.
SAF-T-DATA(tm) Ratings (Provided here for your convenience) Health
Rating: 3 - Severe
Flammability Rating: 2 - Moderate
Reactivity Rating: 2 - Moderate
Contact Rating: 4 - Extreme (Corrosive)
Lab Protective Equip: GOGGLES & SHIELD; LAB COAT & APRON; VENT HOOD;
PROPER GLOVES; CLASS B EXTINGUISHER
Storage Color Code: Red Stripe (Store Separately) Potential Health
Effects
Inhalation:
Vapors are corrosive to the mucous membranes of the upper
respiratory tract. Exposure to vapors may cause irritation of the
nose, throat, and coughing. Exposure to high concentrations may
result in severe damage to the lungs. Symptoms of lung edema are
often delayed and are aggravated by physical effort.
Ingestion:
Corrosive. Causes a burning pain in the stomach, followed by nausea
and vomiting.
Skin Contact:
Corrosive: Does not cause severe burning on contact but can cause
delayed reaction burns. If not removed by washing, the skin may
become reddened and later turn white and wrinkled. Continued skin
contact may cause dermatitis.
Eye Contact:
Corrosive: Contact with the liquid or vapor may produce a burning
sensation and tearing. Redness, pain and blurred vision may be
followed by permanent eye damage. The appearance of eye burns may be
delayed. Irritation effects begin with airborne concentrations as
low as 0.36 mg/m3.
Chronic Exposure:
Repeated and prolonged exposure to vapor may cause irritation of the
skin and chronic eye irritation.
Aggravation of Pre-existing Conditions:
Persons with pre-existing skin disorders or eye problems, or
impaired respiratory function may be more susceptible to the effects
of the substance. Fire:
Flash point: 49C (120F) CC
Autoignition temperature: 316C (601F)
Flammable limits in air % by volume:
lel: 2.7; uel: 10.3
Flammable.
Explosion:
Above flash point, vapor-air mixtures are explosive within flammable
limits noted above. Sealed containers may rupture when heated.
Vapors can flow along surfaces to distant ignition source and flash
back. A violent exothermic reaction occurs with water. Sufficient
heat may be produced to ignite combustible materials. Sensitive to
static discharge.
Fire Extinguishing Media:
Water spray, dry chemical, alcohol foam, or carbon dioxide. Use
water with caution as material reacts with water.
Special Information:
In the event of a fire, wear full protective clothing and NIOSH-approved
self-contained breathing apparatus with full facepiece operated in
the pressure demand or other positive pressure mode. Use water spray
to blanket fire, cool fire exposed containers, and to flush
non-ignited spills or vapors away from fire.
Handling and Storage
Protect against physical damage. Store in a cool, dry
well-ventilated location, away from any area where the fire hazard
may be acute. Outside or detached storage is preferred. Separate
from incompatibles. Containers should be bonded and grounded for
transfers to avoid static sparks. Storage and use areas should be No
Smoking areas. Use non-sparking type tools and equipment, including
explosion proof ventilation. Keep away from water. This material is
corrosive to steel, galvanized iron, copper and copper alloys.
Containers of this material may be hazardous when empty since they
retain product residues (vapors, liquid); observe all warnings and
precautions listed for the product.
Physical and Chemical Properties
Appearance: Clear, colorless liquid.
Odor:Strong acetic odor; good warning properties.
Solubility:Slowly soluble in water (reacts)
Specific Gravity:1.08 @ 15C/4C
pH:No information found.
% Volatiles by volume @ 21C (70F):100
Boiling Point:140C (284F)
Melting Point:-73C (-99F)
Vapor Density (Air=1):3.52
Vapor Pressure (mm Hg):4 @ 20C (68F)
Evaporation Rate (BuAc=1):0.46
Other Information
NFPA Ratings: Health: 3 Flammability: 2 Reactivity: 1
Label Hazard Warning:
DANGER! CORROSIVE. CAUSES BURNS TO ANY AREA OF CONTACT. FLAMMABLE
LIQUID AND VAPOR. WATER REACTIVE. HARMFUL IF SWALLOWED OR INHALED.
VAPOR CAUSES RESPIRATORY TRACT IRRITATION AND SEVERE EYE IRRITATION.
Label Precautions:
Do not get in eyes, on skin, or on clothing.
Do not contact with water.
Do not breathe vapor.
Keep container closed.
Use only with adequate ventilation.
Wash thoroughly after handling.
Keep away from heat, sparks and flame.
Label First Aid:
In case of contact, immediately flush eyes or skin with plenty of
water for at least 15 minutes while removing contaminated clothing
and shoes. Wash clothing before reuse. If inhaled, remove to fresh
air. If not breathing, give artificial respiration. If breathing is
difficult, give oxygen. If swallowed, DO NOT INDUCE VOMITING. Give
large quantities of water. Never give anything by mouth to an
unconscious person. In all cases get medical attention immediately.
Product Use:
Laboratory Reagent.
| |
|
Note /Government
Notification: These chemicals are designated as those that are
used in the manufacture of the controlled substances and are
important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your
country government laws /control substance ACT.
Information: The information on this web page is provided to
help you to work safely, but it is intended to be an overview of
hazards, not a replacement for a full Material Safety Data Sheet
(MSDS). MSDS forms can be downloaded from the web sites of many
chemical suppliers. ,also that the information on the PTCL
Safety web site, where this page was hosted, has been copied
onto many other sites, often without permission. If you have any
doubts about the veracity of the information that you are
viewing, or have any queries, please check the URL that your web
browser displays for this page. If the URL begins "www.tajapi.com/www/Denatonium
Benzoate.htm/" the page is maintained by the Safety Officer in
Physical Chemistry at Oxford University. If not, this page is a
copy made by some other person and we have no responsibility for
it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive
Drug Abuse Prevention and Control Act of 1970.[1] The CSA is the
federal U.S. drug policy under which the manufacture,
importation, possession, use and distribution of certain
substances is regulated. The Act also served as the national
implementing legislation for the Single Convention on Narcotic
Drugs |
|
|
 New Chemicals New Chemicals
Phenyl acetic acid,
3,4-methylenedioxyphenyl-2-propanone,
Piperidine and its salts,
Methylamine,
Propionic anhydride,
Para Methoxy Phenyl Acetone,Para Methoxy Phenyl Acetic Acid,
Benzene,
Benzyl methyl ketone,
3'-Aminoacetophenone,
Ethylamine,
Isosafrole,
Piperonal,
N-methylpseudoephedrine
|