HOME >>
Chemicals
>> Methylamine

|
Methylamine
|
|
Methylamine IUPAC name :
aminomethane
Other names : monomethylamine MMA
Identifiers
CAS number : [74-89-5]
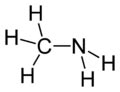
Molecular formula : CH5N
Molar mass : 31.06 g mol−1
Appearance : Colorless Gas
Density : d40.699 (−10.8 °C) / 0.902 g/cm³, 40w/w% in
water
Melting point : −94 °C (179.15 K)
Boiling point : −6 °C (267.2 K)
Solubility in water : 108 g/100 mL (20 °C)
Acidity (pKa) : 10.64 (value for protonated amine, pKaH)
Basicity (pKb) : 3.36
Viscosity : 0.23 cP at 0 °C
Structure : Molecular shape tetrahedral
Dipole moment : 1.31 D (gas)
R-phrases : 11-36/37 (40% solution in water)
Flash point : 8 °C |
Methylamine is the organic compound with a
formula of CH3NH2. This colourless gas is a derivative of ammonia,
wherein one H atom is replaced by a methyl group. It is the simplest
primary amine. It is sold as a solution in methanol, ethanol, THF,
and water, or as the anhydrous gas in pressurized metal containers.
Industrially methylamine is sold in its anhydrous form in
pressurized railcars and tank trailers. It has a strong odour
similar to fish. Methylamine is used as a building block for the
synthesis of many other commercially available compounds.
Production
Methylamine is prepared commercially by the reaction of ammonia with
methanol in the presence of a silicoaluminate catalyst.
Dimethylamine and trimethylamine are coproduced; the reaction
kinetics and reactant ratios determine the ratio of the three
products.
Applications
Methylamine is a good nucleophile as it is highly basic and
unhindered. Its use in organic chemistry is pervasive. Some
reactions involving simple reagents include: with phosgene to methyl
isocyanate, with carbon disulfide and sodium hydroxide to the sodium
methyldithiocarbamate, with chloroform and base to methyl isocyanide
and with ethylene oxide to methylethanolamines.
Representative commercially significant chemicals produced from
methylamine include the pharmaceuticals ephedrine and theophylline,
the pesticides carbofuran, carbaryl, and metham sodium, and the
solvents N-methylformamide and N-methylpyrrolidone. The preparation
of some surfactants and photographic developers require methylamine
as a building block.
Liquid methylamine can be used as a solvent analogous to liquid
ammonia. It shares some of the properties of liquid ammonia, but is
better for dissolving organic substances, in the same way that
methanol is better than water.
Biological chemistry
Methylamine arises naturally as the result of putrefaction
Putrefaction
Putrefaction is the decomposition of animal proteins, especially by
Anaerobic organism, described as putrefying bacteria. Decomposition
is a more general process and is a substrate for methanogenesis
Methanogenesis
Methanogenesis or biomethanation is the formation of methane by
microbes known as methanogens. Organisms capable of producing
methane have been identified only from the Kingdom Archaea, a group
Phylogenetics distinct from both eukaryotes and bacteria, although
many live in close association with anaerobic bacteria. It serves as
a buffering agent.
Buffering agent
A buffering agent adjusts the pH of a solution. The function of a
buffering agent is to drive an acidic or basic solution to a certain
pH state and prevent a change in this pH in the lumen of the
chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryote
organisms that conduct photosynthesis. Chloroplasts capture light
energy to conserve Thermodynamic free energy in the form of
Adenosine triphosphate and reduce NADP to NADPH through a complex
set of processes called photosynthesis in plants, effectively
siphoning off protons that are heading for ATP synthase
An ATP synthase is a general term for an enzyme that can synthesize
adenosine triphosphate from adenosine diphosphate and inorganic
phosphate by using some form of energy.
.
Safety
In toxicology, the median lethal dose, LD50 , or LCt50 of a toxic
substance or radiation is the Dose required to kill half the members
of a tested population (mouse) is 2400 mg/m3. Methylamine is also
controlled as a List 1 substance by the United States Drug
Enforcement Agency (DEA)
Drug Enforcement Administration
The Drug Enforcement Administration is a United States Department of
Justice law enforcement agency tasked with combating War on Drugs
Not only is the DEA the lead agency for domestic enforcement of the
drug policy of the United States , it also has sole responsibility
for coordinating and pursuing U.S. lists methylamine as a precursor
(to methamphetamine.
Methamphetamine is a stimulant and sympathomimetics psychoactive
drug. It is a member of the family of phenylethylamines. The
levorotary levomethamphetamine is an over-the-counter drug and used
in Vicks Inhalers for nasal decongestion and does not possess the
Central nervous system activity of dextro or racemic
methamphetamine.
 Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT. Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Note /Government Notification: N/A

New Chemicals
Phenyl acetic acid,
3,4-methylenedioxyphenyl-2-propanone,
Piperidine and its salts,
Methylamine,
Propionic anhydride,
Para Methoxy Phenyl Acetone,Para Methoxy Phenyl Acetic Acid,
Benzene,
Benzyl methyl ketone,
3'-Aminoacetophenone,
Ethylamine,
Isosafrole,
Piperonal,
N-methylpseudoephedrine
|

![Methylamine CAS number : [74-89-5]](new%20update1/Furfurylamine.jpg)
|


