|
|
HOME >>
API >>
API List 3 >>
Quinine Sulfate

Quinine Sulfate

CAS number 130-95-0
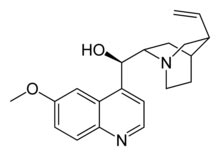
Systematic (IUPAC) name
(R)-(6-methoxyquinolin-4-yl)((2S,4S,8R)- 8-vinylquinuclidin-2-yl)methanol
Identifiers
CAS number 130-95-0
ATC code M09AA01 P01BC01
PubChem 8549
DrugBank APRD00563
ChemSpider 84989
Chemical data
Formula C20H24N2O2
Mol. mass 324.417 g/mol
Physical data
Melt. point 177 °C (351 °F)
Pharmacokinetic data
Bioavailability 76 to 88%
Protein binding ~70%
Metabolism Hepatic (mostly CYP3A4 and CYP2C19-mediated)
Half life ~18 hours
Excretion Renal (20%)
Therapeutic
considerations
Pregnancy cat:. C (USA), D (Au)
Legal status: quinine is now banned in Australia unless specifically for
Malaria due to its tendency to kill blood platelets.
Routes: Oral, intravenous
Quinine is an effective
muscle relaxant, long used by the Quechua Indians of Peru to halt shivering
due to low temperatures. The Peruvians would mix the ground bark of cinchona
trees with sweetened water to offset the bark's bitter taste, thus producing
tonic water.
Quinine has been used in unextracted form by Europeans since at least the
early 17th century. Quinine was first used to treat malaria in Rome in 1631.
During the 17th century, malaria was endemic to the swamps and marshes
surrounding the city of Rome. Malaria was responsible for the death of
several popes, many cardinals and countless common Roman citizens. Most of
the priests trained in Rome had seen malaria victims and were familiar with
the shivering brought on by the febrile phase of the disease. The Jesuit
brother Agostino Salumbrino (1561–1642), an apothecary by training who lived
in Lima, observed the Quechua using the bark of the cinchona tree for that
purpose. While its effect in treating malaria (and hence malaria-induced
shivering) was unrelated to its effect in controlling shivering from rigors,
it was still a successful medicine for malaria. At the first opportunity,
Salumbrino sent a small quantity to Rome to test as a malaria treatment. In
the years that followed, cinchona bark was known as Jesuit's bark and became
one of the most valuable commodities shipped from Peru to Europe.
The form of quinine most effective in treating malaria was found by Charles
Marie de La Condamine in 1737. Quinine was isolated and named in 1820 by
French researchers Pierre Joseph Pelletier and Joseph Bienaimé Caventou. The
name was derived from the original Quechua (Inca) word for the cinchona tree
bark, "quina" or "quina-quina", which roughly means "bark of bark" or "holy
bark". Prior to 1820, the bark was first dried, ground to a fine powder and
then mixed into a liquid (commonly wine) which was then drunk. Large scale
use of quinine as a prophylaxis started around 1850.
Quinine also played a significant role in the colonization of Africa by
Europeans. It has been said that quinine was the prime reason that Africa
ceased to be known as the "white man's grave". A historian has stated that
"it was quinine's efficacy that gave colonists fresh opportunities to swarm
into the Gold Coast, Nigeria and other parts of west Africa".
To maintain their monopoly on cinchona bark, Peru and surrounding countries
began outlawing the export of cinchona seeds and saplings beginning in the
early 19th century. The Dutch government persisted in its attempt to smuggle
the seeds, and by the 1930s Dutch plantations in Java were producing 22
million pounds of cinchona bark, or 97% of the world's quinine production.
During World War II, Allied powers were cut off from their supply of quinine
when the Germans conquered Holland and the Japanese controlled the
Philippines and Indonesia. The United States, however, had managed to obtain
four million cinchona seeds from the Philippines and began operation of
cinchona plantations in Costa Rica. Nonetheless, such supplies came too
late; tens of thousands of U.S. troops in Africa and the South Pacific died
due to the lack of quinine. Despite controlling the supply, the Japanese did
not make effective use of quinine, and thousands of Japanese troops in the
Southwest Pacific died as a result.
Synthetic quinine
Main article: quinine total synthesis
Cinchona trees remain the only economically practical source of quinine.
However, under wartime pressure, research towards its synthetic production
was undertaken. A formal chemical synthesis was accomplished in 1944 by
American chemists R.B. Woodward and W.E. Doering. Since then, several more
efficient quinine total syntheses have been achieved, but none of them can
compete in economic terms with isolation of the alkaloid from natural
sources. The first synthetic organic dye, mauveine, was discovered by
William Henry Perkin in 1856 while he was attempting to synthesize quinine.
Dosing and indication
As of 2006, quinine is no longer recommended by the WHO as first line
treatment for malaria and should be used only when artemesinins are not
available.
Quinine is a basic amine and is therefore always presented as a salt.
Various preparations that exist include the hydrochloride, dihydrochloride,
sulfate, bisulfate and gluconate. This makes quinine dosing complicated
since each of the salts has a different weight.
The following amounts of each form are equal:
-
quinine base 100 mg
-
quinine bisulfate 169 mg
-
quinine dihydrochloride 122
mg
-
quinine hydrochloride 111
mg
-
quinine sulfate (actually
(quinine)2H2SO4∙2H2O) 121 mg
-
quinine gluconate 160 mg.
All quinine salts may be
given orally or intravenously; quinine gluconate may also be given
intramuscularly (IM) or rectally (PR). The main problem with the rectal
route is that the dose can be expelled before it is completely absorbed;
this can be corrected by giving a half dose again.
The IV dose of quinine is 8 mg/kg of quinine base every eight hours; the IM
dose is 12.8 mg/kg of quinine base twice daily; the PR dose is 20 mg/kg of
quinine base twice daily. Treatment should be given for seven days.
The preparations available in the UK are quinine sulfate (200 mg or 300 mg
tablets) and quinine hydrochloride (300 mg/ml for injection). Quinine is not
licensed for IM or PR use in the UK. The adult dose in the UK is 600 mg
quinine dihydrochloride IV or 600 mg quinine sulfate orally every eight
hours. For nocturnal leg cramps, the dosage is 200–300 mg at night.
In the United States, quinine sulfate is commercially available in 324-mg
tablets under the brand name Qualaquin; the adult dose is two tablets every
eight hours. There is no injectable preparation of quinine licensed in the
U.S.: quinidine is used instead.
 |












