|
HOME >>
Chemicals
>>
Chemicals List 1 >>
Ephedrine
Ephedrine
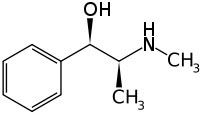
CAS number 299-42-3
 These
terms are used to refer to the same substance derived from the plant
Ephedra is a shrub-like plant that is found in desert regions
in central Asia and other parts of the world. The dried greens of
the plant are used medicinally. Ephedra is a stimulant containing
the herbal form of ephedrine, an FDA-regulated drug found in
over-the-counter asthma medications.Ephedrine alkaloids are
amphetamine-like compounds used in OTC and prescription drugs with
potentially lethal stimulant effects on the central nervous system
and heart. The FDA has received more than 800 reports of adverse
effects associated with use of products containing ephedrine
alkaloid since 1994. These serious adverse effects, include
hypertension (elevated blood pressure), palpitations (rapid heart
rate), neurophathy (nerve damage), myopathy (muscle injury),
psychosis, stroke, memory loss, heart rate irregularities, insomnia,
nervousness, tremors, seizures, heart attacks, and death. The agency
has proposed to prohibit the marketing of dietary supplements
containing 8 milligrams or more of ephedrine alkaloids per serving. These
terms are used to refer to the same substance derived from the plant
Ephedra is a shrub-like plant that is found in desert regions
in central Asia and other parts of the world. The dried greens of
the plant are used medicinally. Ephedra is a stimulant containing
the herbal form of ephedrine, an FDA-regulated drug found in
over-the-counter asthma medications.Ephedrine alkaloids are
amphetamine-like compounds used in OTC and prescription drugs with
potentially lethal stimulant effects on the central nervous system
and heart. The FDA has received more than 800 reports of adverse
effects associated with use of products containing ephedrine
alkaloid since 1994. These serious adverse effects, include
hypertension (elevated blood pressure), palpitations (rapid heart
rate), neurophathy (nerve damage), myopathy (muscle injury),
psychosis, stroke, memory loss, heart rate irregularities, insomnia,
nervousness, tremors, seizures, heart attacks, and death. The agency
has proposed to prohibit the marketing of dietary supplements
containing 8 milligrams or more of ephedrine alkaloids per serving.
Systematic (IUPAC) name
(1R,2S)-2-(methylamino)-1-phenylpropan-1-ol
Identifiers
CAS number 299-42-3
ATC code R01AA03 R03CA02 S01FB02
PubChem 5032
DrugBank DB01364
ChemSpider 8935
Chemical data
Formula C10H15NO
Mol. mass 165.23
SMILES eMolecules & PubChem
Pharmacokinetic data
Bioavailability 85%
Metabolism minimal hepatic
Half life 3–6 hours
Excretion 22-99% renal
Therapeutic considerations
Routes oral, IV, IM, SC
 This
drug should not be used in combination with other stimulant products
(e.g., caffeine), other cough-and-cold products, or as a dietary
supplement for the purpose of weight loss or body building. Doing so
may increase your risk of unlikely but potentially fatal side
effects including: stroke, heart attack, seizures, or severe mental
disorders. This
drug should not be used in combination with other stimulant products
(e.g., caffeine), other cough-and-cold products, or as a dietary
supplement for the purpose of weight loss or body building. Doing so
may increase your risk of unlikely but potentially fatal side
effects including: stroke, heart attack, seizures, or severe mental
disorders.
In addition, dietary supplements containing ephedrine should not
exceed 8 mg as a single ephedrine dose, 24 mg of ephedrine per day
(24 hours), or be given for longer than 7 days, as recommended by
the FDA. Exceeding the recommended ephedrine dose increases your
risk of the side effects noted above. For detailed information,
consult your pharmacist. Check all product labels carefully to see
if they contain ephedrine.
USES: Ephedrine is a central nervous system stimulant used to
treat breathing problems (as a bronchodilator), nasal congestion (as
a decongestant), low blood pressure problems (orthostatic
hypotension), or myasthenia gravis.
OTHER USES: This drug has also been used to treat certain
sleep disorders (narcolepsy), menstrual problems (dysmenorrhea), or
urine-control problems (incontinence or enuresis).
| |
|
Note /Government Notification:
These chemicals are designated as those that are used in the
manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country
government laws /control substance ACT.
Information: The information
on this web page is provided to help you to work safely, but it
is intended to be an overview of hazards, not a replacement for
a full Material Safety Data Sheet (MSDS). MSDS forms can be
downloaded from the web sites of many chemical suppliers. ,also
that the information on the PTCL Safety web site, where this
page was hosted, has been copied onto many other sites, often
without permission. If you have any doubts about the veracity of
the information that you are viewing, or have any queries,
please check the URL that your web browser displays for this
page. If the URL begins "www.tajapi.com/www/Denatonium
Benzoate.htm/" the page is maintained by the Safety Officer in
Physical Chemistry at Oxford University. If not, this page is a
copy made by some other person and we have no responsibility for
it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive
Drug Abuse Prevention and Control Act of 1970.[1] The CSA is the
federal U.S. drug policy under which the manufacture,
importation, possession, use and distribution of certain
substances is regulated. The Act also served as the national
implementing legislation for the Single Convention on Narcotic
Drugs |
|
|
 |



