|
HOME >>
Chemicals
>>
Chemicals List 1 >> Ethylamine
Ethylamine
CAS number
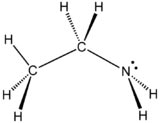
General
Synonyms: monoethylamine, aminoethane, ethanamine Use: synthetic
reagent in the preparation of a wide variety of products
Molecular formula: C2H7N
 MOLECULAR
WEIGHT 45.085 MOLECULAR
WEIGHT 45.085
SPECIFIC VOLUME 8.60 ft3 / lb
CAS No: 75-04-7
EC No: 200-834-7
EU No: 612-002-00-4
Physical data
Appearance: light yellow liquid
Melting point: -81 C
Boiling point: 17 C
Density (g cm-3): 0.689
Flash point: -17 C
Explosion limits: 3.5 - 14%
Autoignition temperature: 385 C
Water solubility: high
Ethylamine is an organic compound with the formula CH3CH2NH2. This
colourless gas has a strong ammonia-like odor. It is miscible with
virtually all solvents and is considered to be a weak base, as is
typical for amines. Ethylamine is widely used in chemical industry
and organic synthesis.
Stability
Stable. Highly flammable. Incompatible with oxidizing agents, alkali
metals, alkaline earth metals, acids, many reactive organic and
inorganic compounds. Reacts with or disssolves most types of paint,
plastic and rubber.
Toxicology
Skin, eye and respiratory irritant. Corrosive - may cause burns to
the eye. Harmful if swallowed or inhaled. Very destructive of mucous
membranes. Typical STEL 20 ppm.
ORL-RAT LDLO 400 mg kg-1
IHL-RAT LCLO 23000 ppm/4h
SKN-RBT LD50 390 mg kg-1
IVN-RBT LDLO 350 mg kg-1Risk phrases R12 R20 R22 R34 R36 R37 R38.
Personal protection
Safety glasses, good ventilation, butyl rubber gloves.
STORAGE
Fireproof. Cool.
PHYSICAL STATE; APPEARANCE:
COLOURLESS COMPRESSED LIQUEFIED GAS , WITH PUNGENT ODOUR.
PHYSICAL DANGERS:
The gas is heavier than air and may travel along the ground; distant
ignition possible.
CHEMICAL DANGERS:
The substance decomposes on heating producing toxic gases including
nitrogen oxides. The solution in water is a strong base, it reacts
violently with acid and is corrosive. Reacts violently with acid,
strong oxidants and organic compounds causing fire and explosion
hazard. Attacks many non-ferrous metals and plastics.
OCCUPATIONAL EXPOSURE LIMITS
TLV: 5 ppm; 9.2 mg/m3 (as TWA) (ACGIH 1993-1994).
TLV (as STEL): 15 ppm; 27.6 mg/m3 (ACGIH 1993-1994).
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by inhalation, through
the skin and by ingestion.
INHALATION RISK:
A harmful concentration of this gas in the air will be reached very
quickly on loss of containment.
EFFECTS OF SHORT-TERM EXPOSURE:
The substance severely irritates the eyes, the skin and the
respiratory tract.
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance may have effects on the kidneys and lungs , resulting
in tissue lesions.
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special
attention should be given to fish.
Ethylamine is also supplied commercially in the form of 70% aqueous
solution (UN number: 2270). Turn leaking cylinder with the leak up
to prevent escape of gas in liquid state.
Ethylamine is a chemical used mainly in the manufacture of dyes,
rayon, rocket propellant, as a fuel additive and in leather-tanning
and cellulose treatment. Ingestion and other exposures to the
chemical can cause various symptoms.
The type and severity of symptoms varies depending on the amount of
chemical involved and the nature of the exposure.
| |
|
Note /Government
Notification: These chemicals are designated as those that are
used in the manufacture of the controlled substances and are
important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your
country government laws /control substance ACT.
Information: The information on this web page is provided to
help you to work safely, but it is intended to be an overview of
hazards, not a replacement for a full Material Safety Data Sheet
(MSDS). MSDS forms can be downloaded from the web sites of many
chemical suppliers. ,also that the information on the PTCL
Safety web site, where this page was hosted, has been copied
onto many other sites, often without permission. If you have any
doubts about the veracity of the information that you are
viewing, or have any queries, please check the URL that your web
browser displays for this page. If the URL begins "www.tajapi.com/www/Denatonium
Benzoate.htm/" the page is maintained by the Safety Officer in
Physical Chemistry at Oxford University. If not, this page is a
copy made by some other person and we have no responsibility for
it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive
Drug Abuse Prevention and Control Act of 1970.[1] The CSA is the
federal U.S. drug policy under which the manufacture,
importation, possession, use and distribution of certain
substances is regulated. The Act also served as the national
implementing legislation for the Single Convention on Narcotic
Drugs |
|
|
 |



